Introduction To Hcooch Ch2 H2o
hcooch ch2 h2o is a simple chemical compound. The full name of hcooch ch2 h2o is methyl formate hydrate. It is made by mixing formic acid, methanol, and water. The formula hcooch ch2 h2o shows the atoms of carbon, hydrogen, and oxygen.
hcooch ch2 h2o is an ester. It is found in liquid form. It has a strong smell like alcohol or fruit. Many people use hcooch ch2 h2o in chemical reactions and lab work.
hcooch ch2 h2o is used in many industries. It helps make medicines, plastics, perfumes, and solvents. It is also used in making pesticides and fuel products.
In chemistry, hcooch ch2 h2o is very useful. It reacts with other chemicals fast. It is easy to work with. Many scientists and engineers choose hcooch ch2 h2o for tests and research.
hcooch ch2 h2o is cheap and useful. It gives fast results. It is also safe when used the right way. That makes hcooch ch2 h2o important in labs and factories.
Chemical Structure And Nomenclature Of Hcooch Ch2 H2o
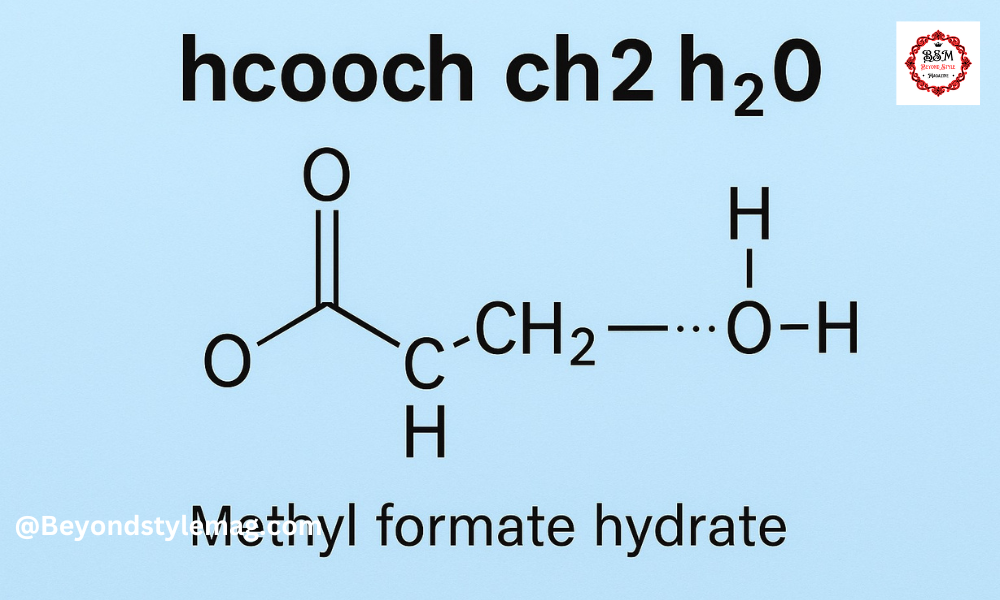
hcooch ch2 h2o is a small and simple molecule. The full molecular formula of hcooch ch2 h2o is made of carbon (C), hydrogen (H), and oxygen (O) atoms. It comes from mixing formic acid, methanol, and water. The molecule contains an ester group, a methyl group, and a water molecule.
The IUPAC name of hcooch ch2 h2o is methyl methanoate monohydrate. In this name:
- “Methyl” comes from the ch2 group,
- “Methanoate” is from the formic acid part (hcooch),
- “Monohydrate” means one water molecule is present (h2o).
This name follows the rules made by IUPAC (International Union of Pure and Applied Chemistry), which gives standard names to all chemical compounds.
The structure of hcooch ch2 h2o is simple. It has a COO group in the center, a CH3 group on one side, and H2O attached through hydrogen bonding. This makes hcooch ch2 h2o both reactive and stable.
A structural diagram of hcooch ch2 h2o would show:
- A carbon atom double bonded to one oxygen,
- Single bonded to another oxygen which is linked to the ch2 group,
- And the h2o molecule connected nearby.
hcooch ch2 h2o has a clean and compact shape, making it easy to use in many lab and industrial settings.
Physical And Chemical Properties Of Hcooch Ch2 H2o
hcooch ch2 h2o is a liquid under normal conditions. It has a clear or slightly yellow color. It smells like fruit or alcohol because it is an ester.
The boiling point of hcooch ch2 h2o is around 32°C to 34°C. It has a low boiling point because it is a light and volatile compound. The melting point is very low, often below -100°C, so it stays liquid in most environments.
hcooch ch2 h2o is soluble in water. It can also dissolve in organic solvents like ether, alcohol, and acetone. This makes it easy to use in chemical reactions.
The density of hcooch ch2 h2o is about 0.97 g/cm³, and the viscosity is low. This means the liquid flows easily and is not thick. It mixes well with many other liquids.
These physical and chemical properties make hcooch ch2 h2o useful in labs and factories.
Esterification And Hydrolysis Reactions Of Hcooch Ch2 H2o
hcooch ch2 h2o is made by a process called esterification. In this process, formic acid reacts with methanol in the presence of acid or heat. When this reaction happens, it forms hcooch ch2 h2o and sometimes water gets added or stays around it.
The reverse process is called hydrolysis. In this reaction, hcooch ch2 h2o breaks into formic acid and methanol again. This happens when water is added and the pH becomes neutral or basic.
The reaction conditions matter. If the temperature is high or if a catalyst is used, the reactions go faster. Acids like sulfuric acid or bases like NaOH can be used to speed up the process.
In industry, both reactions are important. The esterification process is used to make hcooch ch2 h2o in large amounts. The hydrolysis process is used to recover raw materials or to clean up products.
These reactions help make perfumes, solvents, fuels, and other chemical products.
Industrial Applications Of Hcooch Ch2 H2o
hcooch ch2 h2o is used in many industries. It is helpful because it is cheap, fast-reacting, and easy to mix with other liquids. It plays an important role in different types of production and chemical work.
Solvent Use
hcooch ch2 h2o works as a solvent in many chemical reactions. It helps dissolve other compounds. It is often used to make paints, inks, and coatings because it dries fast and mixes well.
Pharmaceutical Industry
In the pharmaceutical field, hcooch ch2 h2o is used to make medicines. It is helpful in the synthesis of drug ingredients. Some drug-making steps use hcooch ch2 h2o to carry other chemicals or to remove unwanted parts.
Agriculture
hcooch ch2 h2o is used in making pesticides and fertilizers. It helps carry active ingredients in sprays. Because of its fast action, it is good for short-term pest control. It also helps in cleaning tools used in farming.
Textile And Leather Processing
In textile and leather industries, hcooch ch2 h2o is used in dyeing, cleaning, and tanning. It helps remove oil and dirt from fabrics. It also helps in softening leather and fixing dyes to fibers.
Food Industry
hcooch ch2 h2o is sometimes used as a flavoring agent. Its smell is sweet, like fruit or rum. It is also used as a preservative in small, safe amounts to keep foods fresh.
Plastic And Polymer Manufacturing
In the plastic industry, hcooch ch2 h2o acts as a plasticizer. It helps make plastics more flexible and smooth. It is also used in the process of making some resins and synthetic materials.
Environmental Impact And Safety Measures Of Hcooch Ch2 H2o
hcooch ch2 h2o has both good and bad effects on the environment. It is important to know how to use it safely.
Biodegradability
hcooch ch2 h2o breaks down easily in air and water. That means it is biodegradable. It does not stay long in nature. This makes hcooch ch2 h2o better for the environment than many other chemicals.
Toxicity Levels
In small amounts, hcooch ch2 h2o is not very toxic to humans. But in large amounts, it can cause eye and skin irritation. Breathing high levels of hcooch ch2 h2o may lead to headache or dizziness. It is harmful to some water animals if dumped into rivers or lakes.
Handling Precautions
Always handle hcooch ch2 h2o in a well-ventilated area. Use gloves, masks, and goggles. Keep it in a sealed container and store it away from heat and flames. Do not mix hcooch ch2 h2o with strong acids or bases unless needed.
Regulatory Guidelines
Different countries have rules for using hcooch ch2 h2o. Agencies like OSHA and EPA set safety limits. Labels must show the right warnings. Waste must be disposed of in safe ways. Always follow the local laws for chemical use.
Future Prospects In Green Chemistry Using Hcooch Ch2 H2o
hcooch ch2 h2o has strong potential in the world of green chemistry. Scientists are working on new ways to use it in safe and eco-friendly products.
Renewable Production Methods
One goal is to make hcooch ch2 h2o using natural and renewable sources. Plants and bio-waste can help produce formic acid and methanol, which are used to make hcooch ch2 h2o. This lowers the need for fossil fuels.
Energy Storage Applications
Researchers are looking at hcooch ch2 h2o for energy storage. Some tests show it can help carry hydrogen or act as a fuel in small energy cells. This may help store solar and wind energy in the future.
Biodegradable Materials
hcooch ch2 h2o can be used to make eco-friendly plastics. These plastics break down in nature and do not harm the planet. It may help create green packaging and biodegradable containers.
Research And Development
Many labs are studying new ways to use hcooch ch2 h2o. New uses may include medicine, battery systems, and eco-cleaning products. This research shows that hcooch ch2 h2o is not just useful today, but also in the future.
Conclusion
This article explained many important things about hcooch ch2 h2o. We learned that hcooch ch2 h2o is a simple chemical called methyl formate hydrate. It has a clear molecular structure and is easy to use in labs and industry.
We saw its physical and chemical properties like low boiling point and good solubility. The article also described how hcooch ch2 h2o forms in esterification and breaks down in hydrolysis. Many industries use hcooch ch2 h2o as a solvent, in medicine, agriculture, textiles, food, and plastics.
Safety and environmental care are important when working with hcooch ch2 h2o. It is biodegradable but needs careful handling. Lastly, future research shows that hcooch ch2 h2o can help in green chemistry, energy storage, and biodegradable materials.
hcooch ch2 h2o is a useful and promising compound with many current and future roles.
FAQ’s:
What Is Hcooch Ch2 H2o Used For?
It is used as a solvent, in making medicines, pesticides, plastics, and perfumes.
Is Hcooch Ch2 H2o Safe?
It is safe if handled properly. Avoid skin contact and breathing in large amounts.
Can Hcooch Ch2 H2o Dissolve In Water?
Yes, hcooch ch2 h2o dissolves well in water and many organic solvents.
How Is Hcooch Ch2 H2o Made?
It is made by esterification of formic acid and methanol.
Does Hcooch Ch2 H2o Harm The Environment?
It breaks down quickly and is biodegradable but should not be released in large amounts.
If you found this article helpful, visit my website to read more articles like this.
Complete Guide To Fintechzoom.com Stoxx 600: Market Updates, Analysis, And Investment Tips